ABC - Department for Genetics - Plant genomics and plant-microbe interaction group
Research interest
In nature, plants are continuously challenged by microorganisms affecting their growth and production. These interactions can be detrimental (pathogenic), neutral (saprophytic), or beneficial (symbiotic). The research interest of our group is the genetic dissection of plant-microbe interactions and the aim of our research is to identify and analyze the function of plant genes involved in symbiotic and pathogenic interactions of plants. We work on two model systems; the symbiotic interaction is analyzed between Medicago truncatula and Sinorhizobium meliloti and we study the pathogenic interactions of pepper (Capsicum annuum).
Legumes compose the third largest family of flowering plants. Medicago truncatula and other leguminous plants are able to establish nitrogen-fixing symbiotic associations with soil bacteria belonging to the genus rhizobia. The legume-rhizobial symbiosis accounts for a significant proportion of biological nitrogen fixation worldwide. Symbiotic nitrogen fixation takes place in specialized organs on the root, termed nodules. The aim of our research is to reveal the molecular steps of the nodule invasion and function to better understand the molecular basis of symbiotic nitrogen fixation.
Environmental-friendly plant cultivation with limited use of chemicals against stress factors (biotic and abiotic) causing decrease in production is a demand in modern agriculture. To reduce the use of pesticides, the application of resistance genes is particularly important in plant protection. MAS (Marker Assisted Selection) is an indirect selection process where a trait of interest is selected based on linked genetic markers. DNA sequences of these resistance genes can be the templates for the generation of gene specific marker. The application of these markers makes more efficient the traditional breeding, follow up genetic inheritance of specific traits, selection of breeding lines.
Current research projects
Genetic dissection of the invasion and functioning nitrogen-fixing nodules
Genetic analysis of legumes
Mapping and cloning of resistance genes from pepper – molecular applications for multi-resistant pepper lines
Genetic dissection of the nodule invasion and functioning
Symbiotic nitrogen fixation takes place in specialized organs termed nodules, which are initiated within a specific symbiotic sensitive region of root. In nodule cells, the bacteria are encompassed by plant derived peribacteroid membranes and these cytoplasmic structures containing rhizobia are referred as symbiosomes. In the symbiosomes the bacteria undergo morphological changes and metabolic differentiation to transform into their symbiotic state termed bacteroids. The symbiosome is the site of nitrogen fixation and functions in nutrient and signal exchange between the two symbionts. The research of our group focuses on the identification, molecular and biochemical characterization of plant components essential in the later stages of nodule development, rhizobial invasion and symbiosome development and function. Bacterial and plant symbiotic mutants provide excellent tools to dissect the interaction between the two partners and to analyze the development of the symbiotic nodule. The identification of key components in bacterial release, endocytosis and symbiosome development will help us to better understand the symbiotic interaction.
We are currently characterizing and analyzing M. truncatula mutants in which nodule formation is arrested in the later stages of nodule organogenesis and that are impaired in proper functioning of the symbiotic nodules. In order to assess the actual function of the genes (Fix genes) conditioning the mutant phenotype and characterize their gene products, the Fix genes will be identified either by transcript based or positional cloning.
Mutant plant impaired in the symbiotic nitrogen fixation shows the symptoms of nitrogen deficiency under symbiotic conditions (low nitrogen medium).
In the last two decades, Medicago truncatula has become one of the model legumes due to its advantageous characteristics, such as small and diploid genome, self-fertility and short life cycle. Enormous genomic information has been collected and various genomic resources have been developed for this model species. Besides focusing on the study of symbiotic nitrogen fixation our mission is the exploitation of genetic resources of model legume organism to develop genomic tools for related crop legume species. In order to address this task we focus on the analysis of the synteny of genetic maps of M. truncatula, alfalfa and pea. One of the most interesting question to us, how we can use the M. truncatula genomic resource to analyze and improve specific traits of cultivated alfalfa. Due to its allogamous nature, alfalfa plants are highly heterozygous and the heterozygosity at several loci in the genome is required for the crop values of alfalfa. Our aim is to identify the loci which heterozygous state is advantageous or essential for the vigor and productivity of alfalfa lines.
Molecular mapping and map based cloning of resistance genes from pepper
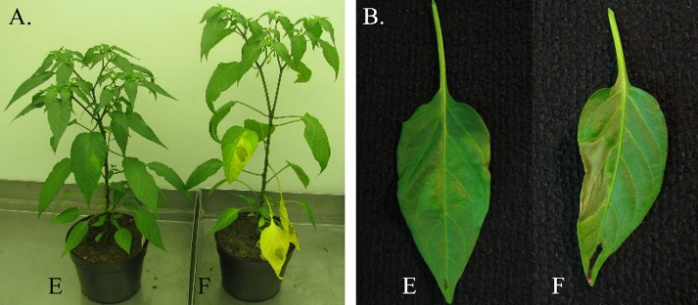
The most devastating pathogens of pepper on the field are Xanthomonas campestris pv. vesicatoria (Xcv), and Tomato Spotted Wilt Virus, (TSWV), Meloidogyne spp (Me) in greenhouses. The main quest is to develop the genetic map of pepper focusing on the resistant genes against Xcv, Me and TSWV infection. Based on the improved linkage map and flanking markers of contigs, the alleles of the resistance/susceptibility genes will be isolated. DNA and amino acid sequence of resistant alleles could highlight micro-evolutional changes and could lead to better understanding the bacterial, viral or parasite disease strategies. The development of closely linked or gene specific markers could be used in marker assisted selection (MAS) to generate multi resistant pepper varieties that provide the most effective method contrary to the chemical control of the disease. The study will give background information on molecular, biochemical and physiological mechanisms involved in plant-pathogen interaction.
Xanthomonas infection causes symptoms on the leaves (F) of susceptible plants (E - resistant plant).
Current research funding:
The identification and analysis of genes controlled by the regulator of symbiosome differentiation (RSD) transcription factor during Medicago truncatula nodule development. ICGEB CRP/HUN17-03
The functional study of nodule-specific cystein-rich (NCR) peptide genes required for bacterial differentiation in Medicago truncatula. OTKA K-119652
The functional analysis of the indispensable Medicago truncatula nodule-specific cysteine-rich (NCR) peptide family required for rhizobium terminal bacteroid differentiation. OTKA PD- 121110
Profit maximalization in symbiosis? Gene for gene interactions in the Medicago-Sinorhizobium partnership. OTKA K-120300
Molecular and functional characterization of genes controlling resistance to tomato spotted wilt virus and bacterial spot in pepper (Capsicum annuum L.). – OTKA K-111935
Recently terminated research funding
Identification and analysis of genetic loci that cause heterosis in crop plants - OTKA105170
ABSTRESS - Improving the resistant of legume crops to combined abiotic and biotic stress - FP7/KBBE/2011 289562
Molecular characterization of a nitrogen fixation deficient Medicago truncatula mutant showing vigorous pathogen response OTKA PD-104334
Molecular factors changing the fate of plant-microbe interactions - OTKA106068 (Consortional assoc. grant)
LEGUMICS – Expanding the genomic tools for legumes and demonstrating their power in roots and symbiotic nodule development studies - TÉT_10-12011-0397
Staff






Publications
Publication (2005-present)
Laffont, C, Huault, E, Gautrat, P, Endre, G, Kalo, P, Bourion, V, Duc, G, Frugier, F (2019) Systemic regulation of symbiotic nodulation. Plant Physiol. (in press) https://doi.org/10.1104/pp.18.01588
Rajlakshmi Das, D, Horváth, B, Kundu, A, Kaló, P, DasGupta M (2019) Functional conservation of CYCLOPS in crack entry legume Arachis hypogaea. Plant Science 281: 232-241
Ellis, N, Hattori, C, Cheema, J, Donarski, J, Charlton, A, Dickinson, M, Venditti, G, Kalo, P, Szabo, Z, Kiss, GB, Domoney, C (2018) NMR Metabolomics Defining Genetic Variation in Pea Seed Metabolites. Frontiers in Plant Science 9:1022 DOI: 10.3389/fpls.2018.0102
Domonkos, Á, Kovacs, S, Gombar, A, Kiss, E, Horvath, B, Kovats, G, Farkas, A, Toth, M, Ayaydin, F, Boka, K, Fodor, L, Ratet, P, Kereszt, A, Endre, G, Kaló P (2017) NAD1 controls defense-like responses in Medicago truncatula symbiotic nitrogen fixing nodules following rhizobial colonization in a BacA-independent manner. Genes 8:387
Wang, Q, Yanga, S, Liu, J, Terecskei, K, Ábrahám, E, Gombár, A, Domonkos, Á, Szűcs, A, Körmöczi, P, Wang, T, Fodor, L, Mao, L, Fei, Z, Kondorosi, É, Kaló, P, Kereszt, A and Zhu, H (2017) Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula Proc Nat Acad Sci 114: 6854-6859
Marincs, F, Nagy, T, Miró, K, Kollár, Z, Barta, E, Kaló, P (2017) Large-scale amplicon sequencing of the SP3D gene responsible for fruit-yield heterosis in tomato. Plant Gene 9: 45–49
Horváth B, Domonkos Á, Kereszt A, Szűcs A, Ábrahám E, Ayaydin F, Bóka K, Chen Y, Chen R, Murray JD, Udvardi MK, Kondorosi É, Kaló P (2015) Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc Nat Acad Sci 112:15232-15237
Domonkos, A, Horváth, B, Marsh, JF, Halász, G, Ayaydin, F,Oldroyd, GED, Kaló, P (2013) The identification of novel loci required for appropriate nodule development in Medicago truncatula. BMC Plant Biology 13: 157 DOI: 10.1186/1471-2229-13-157
Horváth B, Yeun LH, Domonkos, Á, Halász G, Gobbato E, Ayaydin F, Miró K, Hirsch S, Sun J, Tadege M, Ratet P, Mysore K, Ané JM, Oldroyd GED and Kaló P (2011) Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol Plant-Microbe Interaction, 24:1345-1358
Rispail N, Kaló P, Kiss GB, Ellis THN, Gallardo K, Thompson RD, Prats E, Larrainzar E, Ladrera R, González EM, Arrese-Igor C, Ferguson BJ, Gresshoff PM, Rubiales D (2010) Model legumes contribute to faba bean breeding. Field Crops Research, 115:253-269.
Bajkan, S, Varadi, G, Balogh, M, Domonkos, A, Kiss, GB, Kovacs, L, Lehoczki, E. (2010) Conserved structure of the chloroplast-DNA encoded D1 protein is essential for effective photoprotection via non-photochemical thermal dissipation in higher plants. Molecular Genetics and Genomics 284: 55-63
Kiss E, Oláh B, Kaló P, Morales M, Heckmann AB, Borbola A, Lózsa A, Kontár K, Middleton P, Downie JA, Oldroyd GED and Endre G (2009) LIN, a novel type of U-box/WD40 protein, controls early infection by rhizobia in legumes. Plant Physiology, 151: 1239-1249
Ding Y., Kaló P, Yendrek C, Sun, J, Liang, Y, Marsh JF, Harris JM and Oldroyd GED (2008) Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20:2681-2695.
Dalmadi A, Kaló P, Jakab J, Saskői A, Petrovics T, Deák G, Kiss GB (2008) Dwarf plants of diploid Medicago sativa carry a mutation in the gibberellin 3-β-hydroxylase gene. Plant Cell Reports, 27: 1271-1279
Middleton, P, Jakab, J, Penmetsa, RV, Starker, CG, Doll, J., Kaló, P, Prabhu, R, Marsh, JF, Mitra, RM, Kereszt. A, Dudas, B, VandenBosch, K, Long, SR, Cook, DR, Kiss, GB and Oldroyd, GED (2007) An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19 (4): 1221-1234
Szűcs, A, Dorjgotov, D, Ötvös, K, Fodor, C, Domoki, M, Györgyey, J, Kaló, P, Kiss, GB, Dudits, D, Fehér, A (2006) Characterization of three Rop GTPase genes of alfalfa (Medicago sativa L.). Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1759:108-115.
Kevei, Z, Seres, A, Kereszt A, Kaló, P, Kiss, P, Tóth, G, Endre, G, Kiss, GB (2005) Significant microsynteny with new evolutionary highlights is detected between Arabidopsis and legume model plants despite the lack of macrosynteny. Mol Genet Genomics 274: 644–657.
Kaló, P, Gleason, C, Edwards, A, Marsh, J, Mitra, RM, Hirsch, S, Jakab, J, Sims, S, Long, SR, Rogers, J, Kiss, GB, Downie JA, and Oldroyd GED (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308:1786-1789.